Molecular capsule catalysis: Ready to address current challenges in synthetic organic chemistry?
Self-assembled molecular capsules are closed host structures that form spontaneously when their building blocks are mixed in a suitable solvent.[1] As they feature a defined binding pocket suitable for substrate uptake, they are regarded as simple enzyme pocket mimics. Although their potential as catalysts has been explored since their discovery, applications that solve current challenges in synthetic organic chemistry have remained limited.
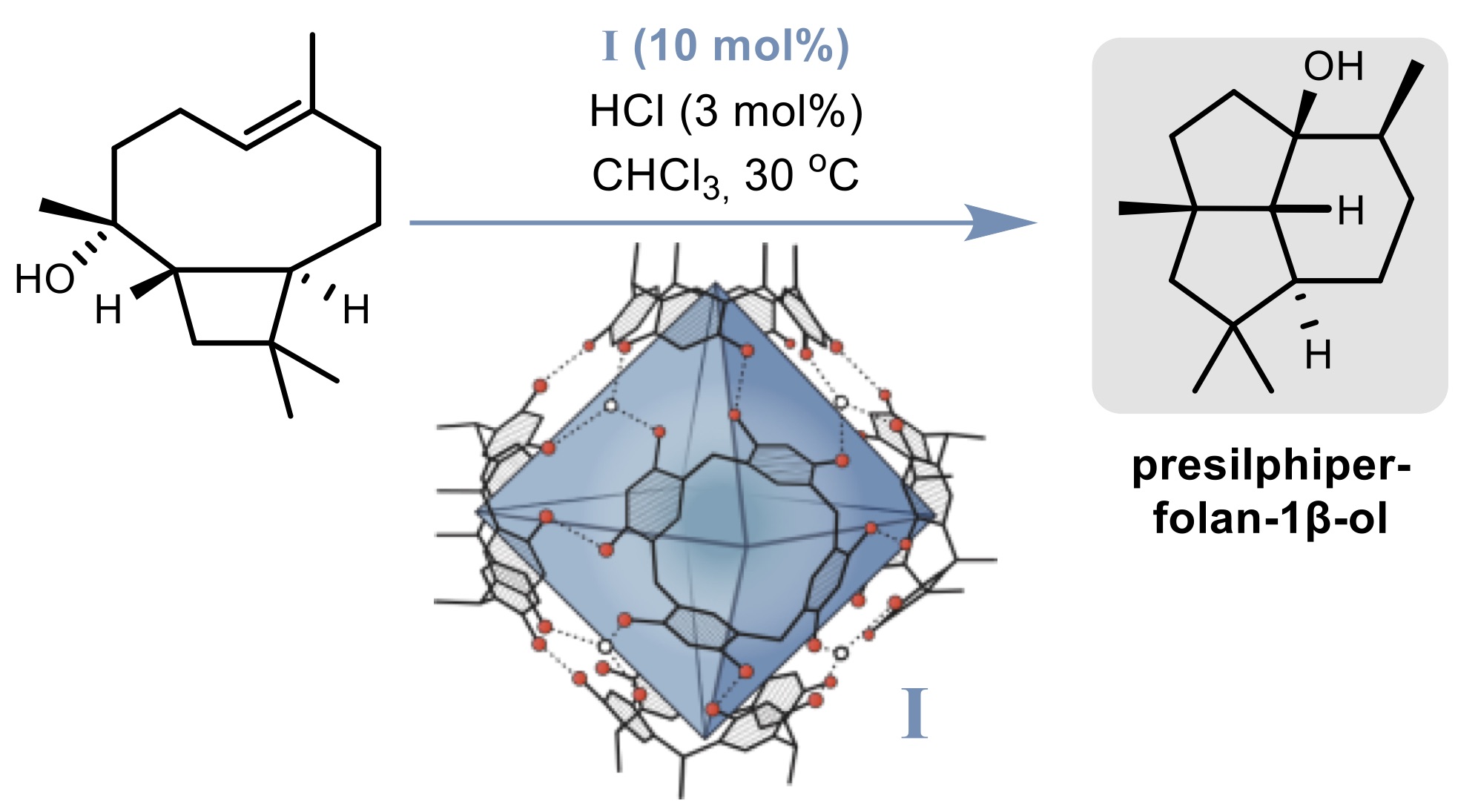
In this presentation, I will present some of our recently published and unpublished solutions for current synthetic challenges that are difficult to address with alternative tools. For instance, we were able to synthesize the tricyclic sesquiterpene natural product presilphiperfolan-1β-ol in only four steps, with the key cyclization (see below) taking place inside the supramolecular catalyst I.[2] This natural product was previously only available via a long synthetic sequence (13 linear steps),[3] demonstrating the potential of molecular capsule catalysis for natural product synthesis. Interestingly, extensive control experiments in solution indicated that the key step is not possible in solution utilizing regular Lewis or Brønsted acid catalysis. Several further examples of catalysis inside capsule I, which was reported already more than 20 years ago by the Atwood group,[4] will be presented.
[1] M. Conn, J. Rebek, Chem. Rev. 1997, 97, 1647.
[2] L.-D. Syntrivanis, I. Némethová, D. Schmid, S. Levi, A. Prescimone, F. Bissegger, D. T. Major, K. Tiefenbacher J. Am. Chem. Soc. 2020, 142, 5894.
[3] A.Y. Hong, B.M. Stoltz Angew. Chem. Int. Ed. 2012, 51, 9674.
[4] L. R. MacGillivray, J. L. Atwood Nature 1997, 389, 469.