Identity of the Cu(II) Active Site on Alumina for the Stepwise Selective Conversion of Methane to Methanol
The direct conversion of methane into methanol is one of the major challenges of modern catalysis, since it has the potential for a transition from a fossil fuel-based economy to more renewable energies. However, this direct transformation is challenging due to the increased reactivity of the desired product compared to methane, leading to the formation of overoxidized products.[1] Among recent materials explored, Cu-exchange zeolites have shown the greatest potential for the selective oxidation of methane to methanol under stepwise reaction conditions.[2] Recent economic studies have shown the economic limitation of these materials, among them the high price of zeolite and long cycling times.[3] In that context, cheap and readily available supports such as oxides, must be further explored.
Surface organometallic chemistry (SOMC)[4] combined with thermolytic molecular precursors (TMP)[5] has emerged as a powerful approach to generate isolated metal sites with controlled nuclearity and oxidation state on oxide supports, allowing to derive structure – reactivity relationships for a wide range of reactions.[6] Employing this methodology, we generated well-dispersed monomeric Cu(II) sites on a series of transitional aluminas (γ-,η-, θ- and α-Al2O3) and were able to rationalize reactivity observed (Figure 1a) to specific properties of the support. The identity of the reactive monomeric Cu(II) site could be unambiguously determined by its EPR spectroscopic signature (Figure 1b). Combination of advanced spectroscopic techniques (EPR, IR, XAS) with DFT calculation allowed the assignment of the reactive Cu(II) site to a tricoordinated Cu(II) present on the (110) facet of alumina.
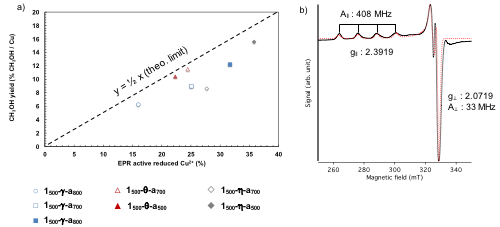
Figure 1. a) methanol yield vs. Cu(II) reduction for the alumina series b) EPR signature of the reactive monomeric Cu(II) species.
[1] a) Crabtree et al. Chem. Rev. 1995, 95, 987-1007 b) Labinger et al. Nature 2002, 417, 507-514 c) Schwarz et al. Angew. Chemie Int. Ed. 2011, 50, 10096-10115 c) Ravi et al. Angew. Chem. Int. Ed. 2017, 56, 16464-16483 d) Meyet et al. Angew. Chemie Int. Ed. 2019, 58, 9841-9845
[2] Groothaert et al. J. Am. Chem. Soc. 2005, 127, 1394-1395
[3] Lange et al. Ind. Eng. Chem. Res. 2019, 58, 8674-8680
[4] Copéret et al. Angew. Chem. Int. Ed. 2018, 57, 6398-6440
[5] Fujdala et al. Topics Organometal. Chem. 2005, 16, 69–11
[6] Copéret et al. Acc. Chem. Res. 2019 52, 1697-1708