Understanding Catalysis in Iron Heme Metalloproteins Using Non-Canonical Amino Acids
Non-canonical amino acid ligands are useful for fine-tuning the catalytic properties of metalloenzymes. We showed that recombinant replacement of the histidine ligand proximal to heme in myoglobin with Nδ-methylhistidine enhances the protein’s promiscuous carbene transfer chemistry, enabling efficient styrene cyclopropanation in the absence of reductant, even under aerobic conditions1. The mutant protein’s reduced sensitivity to oxygen enabled us to capture and characterize a reactive carbenoid adduct by UV/vis spectroscopy, EPR spectroscopy and X-ray crystallography. Together with another structure recently published by the Arnold group2, these two structures represent the first carbenoid structures characterized in a protein. Elaborating on this approach, we further expanded the panel of histidine analogs accessible for incorporation into proteins via stop codon suppression. These analogs include 4- and 5-thiazolyl alanine as well as 3-thienyl alanine which can coordinate the iron heme either via nitrogen or sulfur. Characterization of the reactivity of these proteins in three carbene transfer model reactions– N-H insertion, S-H insertion and cyclopropanation– shows that the non-canonical amino acid 5-thiazolyl alanine is a privileged proximal ligand, as the corresponding myoglobin derivatives outperform other enzymes under most tested reaction conditions. Our findings suggest that Fe(III) catalysis is possible in carbene transfer reactions catalyzed by iron heme proteins, previously Fe(II) catalysis was the most widely accepted mechanistic proposition. Overall, our studies show that non-canonical amino acids can enhance catalytic performance, enable novel chemistries and reveal reactive intermediates.
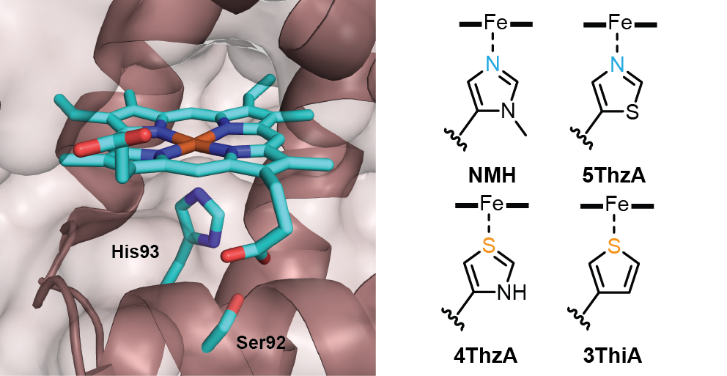
Fig: Active site of myoglobin showing ligation of the heme cofactor by His93. Non canonical amino acids incorporated in place of histidine are Nδ-methylhistidine (NMH), 5-thiazolyl alanine (5Thz), 4-thiazolyl alanine (4Thz) and 3-thienyl alanine (3ThiA).
[1] Takahiro Hayashi, Matthias Tinzl, et al., Nature Catalysis, 2018, 1, 578–584.
[2] Russel D. Lewis, et al., Proceedings of the National Academy of Sciences, 2011, 115, 7308–7313