Atomistic simulations of catalytic confined environments from supramolecular systems to biomass conversion.
In recent years, new materials and processes opened the way to novel catalytic pathways. The main feature of these systems is the simultaneous presence of a liquid substrate/solvent solution confined in nanosized catalytic environments. These processes are highly relevant because they can potentially revolutionize industrial production for a more sustainable chemical industry, ranging from fine chemistry[1] up to biomass conversion[2].Enhanced sampling molecular dynamics simulations with realistic models of condensed phase systems are in able to describe accurately the behavior of complex catalytic interfaces and reactions.
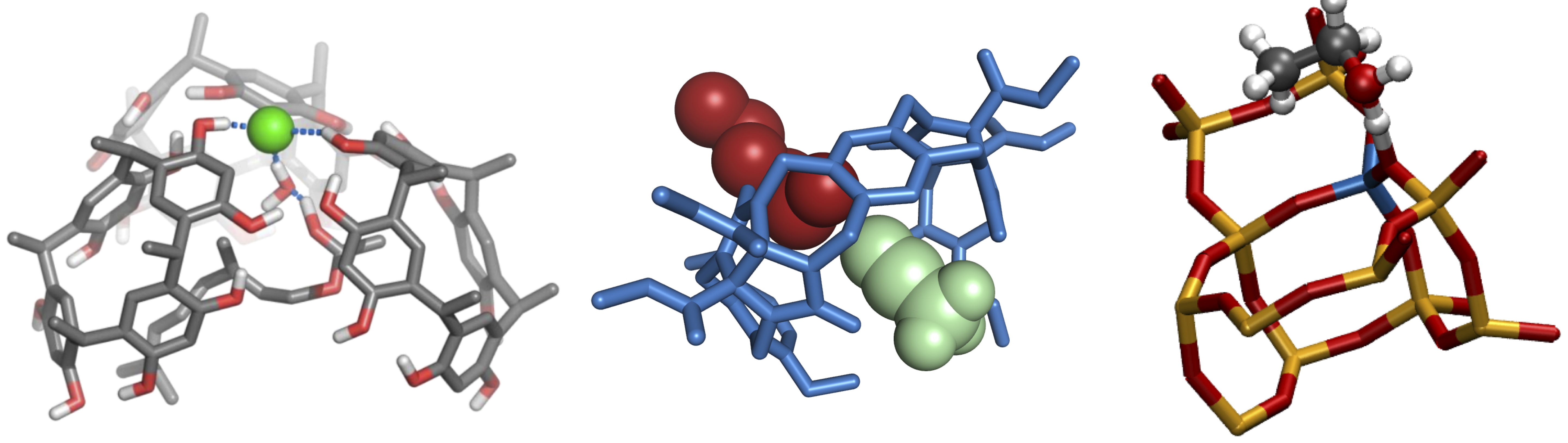
Here, we report the study of two types of catalysis characterized by well-defined cavities hosting a limited number of solvent and substrate molecules: 1) organic reactions in hexameric calixarene nanocapsules and molecular tweezers and 2) liquid-phase biomass conversion over zeolites.
For the first project we characterized the acid-base properties of an hexameric rescorcinarene capsule catalyzing terpene cyclization[3], and studied the conformational changes in the binding mode of an alkylammonium cation within a glycouril based molecular tweezers[4] leading to highly specific catalytic activity. Whereas, for the second project we aimed at understanding the process of ethanol dehydration in the acidic zeolites chabazite. For both research topics, metadynamics simulations have been used in combination with recently developed methods for studying activated processes relevant to catalysis[5-7]. This allowed us to understand the complex dynamics of different catalytic reactions and shed light on the atomistic behavior of these complex molecular systems.
[1] J. Meeuwissen, J. N. H. Reek, Nat. Chem. 2010, 2, 615–621.
[2] T. Ennaert, J. Van Aelst, J. Dijkmans, R. De Clercq, W. Schutyser, M. Dusselier, D. Verboekend, B. F. Sels, Chem. Soc. Rev. 2016, 45, 584–611.
[3] S. Merget, L. Catti, G. Piccini, K. Tiefenbacher, J. Am. Chem. Soc. 2020 142, 9, 4400-4410.
[4] M. Knezevic, M. Heilmann, G. Piccini, Tiefenbacher K. Angew. Chem. Int. Ed. 2020 Accepted article.
[5] D. Mendels, G. Piccini, M. Parrinello, J. Phys. Chem. Lett. 2018, 9 (11), 2776–2781.
[6] E. Grifoni, G. Piccini, M. Parrinello, Proc. Natl. Ac. Sci. 2019, 116 (10), 4054–4057
[7] G. Piccini, M. Parrinello, J. Phys. Chem. Lett. 2019, 10 (13), 3727–3731.