Iridium-catalyzed acid-assisted asymmetric hydrogenation of oximes to hydroxylamines
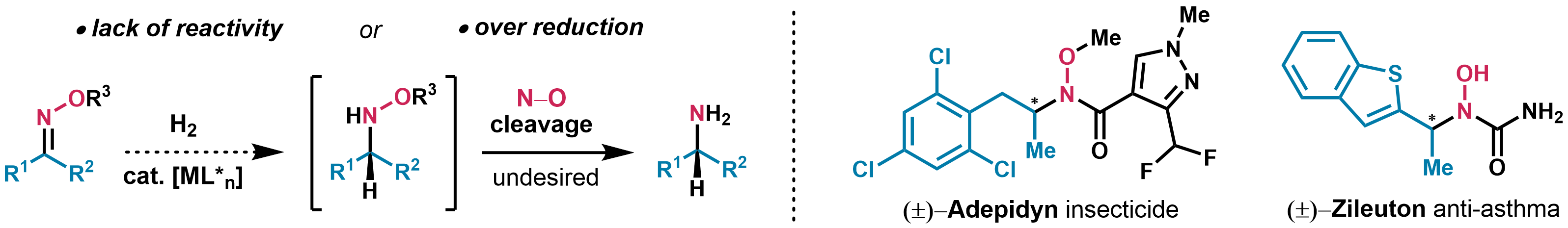
Asymmetric hydrogenation with homogeneous transition metal-catalysts is one of the most efficient methods for the synthesis of chiral building blocks at industrial scale.[1] The selective reduction of an oxime to the corresponding chiral hydroxylamine derivative remains elusive. These substrates are often inert, and when reactivity is observed, undesired reductive cleavage of the labile N‒O bond leads to primary amines.[2] Current bioactive N‒O compounds (e.g. Adepidyn, Zileuton) are marketed as racemates. A practical asymmetric synthesis would facilitate incorporation of chiral 3D hydroxylamine scaffolds as design elements in drug discovery.
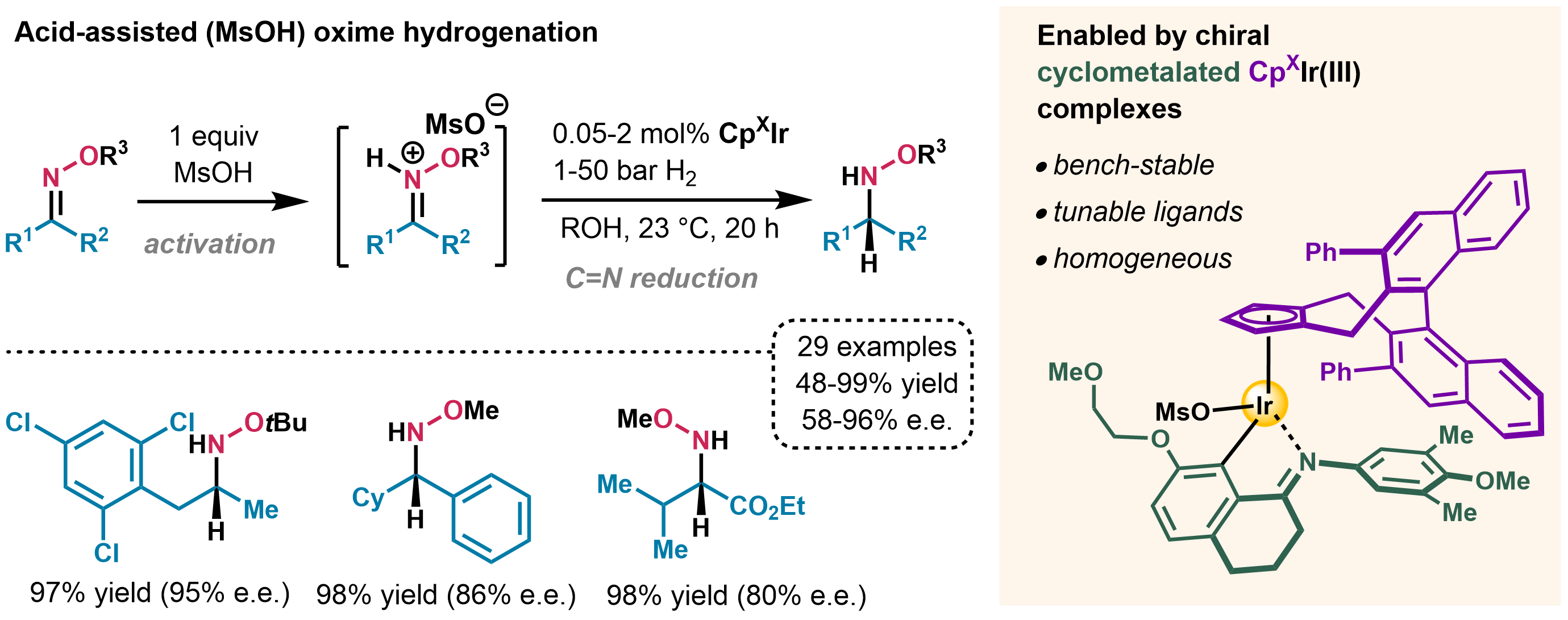
We developed a robust cyclometalated iridium(III) complex bearing a chiral cyclopentadienyl ligand (CpX) as an efficient catalyst for oxime hydrogenation under highly acidic conditions.[3] The reaction is fully chemoselective towards reduction of the C=N bond, showing no cleavage of the N‒O bond. Valuable N-alkoxy amines can be accessed at room temperature in catalyst turnover numbers up to 4000 and enantiomeric ratios up to 98:2. These findings may inspire future metal-catalyzed enantioselective hydrogenations of challenging substrates.
[1] David J. Ager, André H. M. de Vries, Johannes G. de Vries, Chem. Soc. Rev., 2012, 41, 3340-3380.
[2] Anna M. Maj, Isabelle Suisse, Francine Agbossou-Niedercorn, Tetrahedron: Asymmetry, 2016, 27, 268-273.
[3] Josep Mas-Roselló, Tomas Smejkal, Nicolai Cramer, Science, in press.