Synthesis and biological investigation of new (–)-zampanolide analogues
The continuous evaluation of new cytotoxic natural products offers significant potential for the discovery of new antitumor agents, which may overcome the multi-drug-resistance (MDR) problem. (–)-Zampanolide[1,2] is a marine macrolide, which exhibits low nM cytotoxicity against both drug-sensitive and MDR cancer cell lines.[3]
In 2013, a crystal structure of the complex between αβ-tubulin and (–)-zampanolide has been characterized.[4] Based on the crystal structure and existing SAR data, we have prepared new (–)-zampanolide analogues with reduced structural complexity and we have investigated the effects of these structural changes on microtubule-binding affinity and cellular potency.
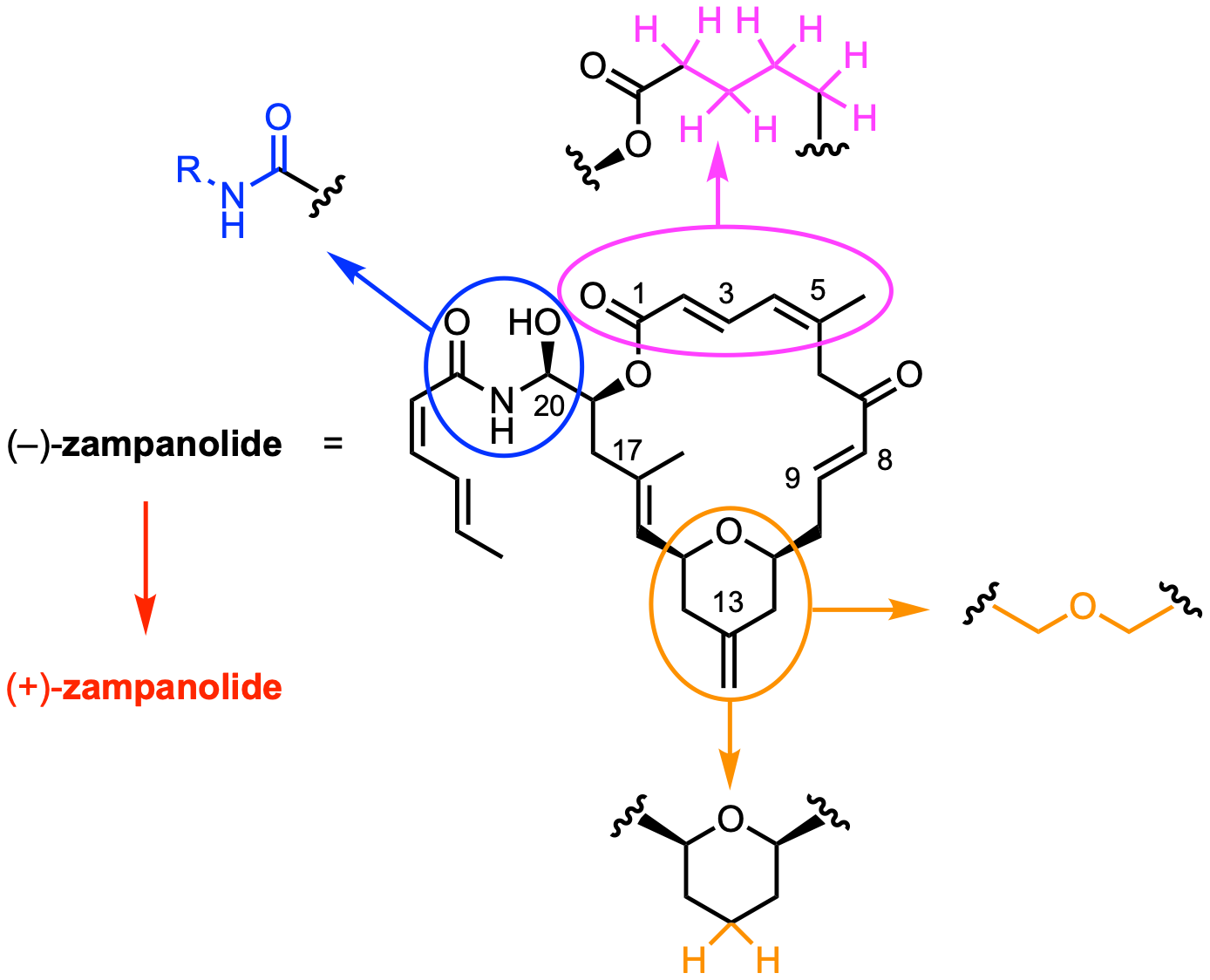
For one analogue, we were also able to obtain an X-ray crystal structure in complex with β-tubulin. While this analogue had retained the potency of (–)-zampanolide almost completely, significant differences were found between the binding mode of the natural product and its modified congener. Finally, we have evaluated basic pharmacokinetic properties of selected compounds in vitro, as a basis for potential future in vivo applications.
[1] J. Tanaka, T. Higa, Tetrahedron Lett. 1996, 37, 5535–5538.
[2] J. J. Field, A. J. Singh, A. Kanakkanthara, T. Halafihi, P. T. Northcote, J. H. Miller, J. Med. Chem. 2009, 52, 7328–7332.
[3] Q.-H. Chen, D. G. I. Kingston, Nat. Prod. Rep. 2014, 31, 1202–1226.
[4] A. E. Prota, K. Bargsten, D. Zurwerra, J. J. Field, J. F. Díaz, K. H. Altmann, M. O. Steinmetz, Science (80-. ). 2013, 339, 587–590.