Simulating the Ghost: Quantum Dynamics of Solvated Electron
The nature of bulk hydrated electron has been a challenge for both experiment and theory due to its short life time and high reactivity, and the need for a high-level of electronic structure theory to achieve predictive accuracy. The lack of a classical atomistic structural formula makes it exceedingly difficult to model the solvated electron using conventional empirical force fields, which describe the system in terms of interactions between point particles associated with atomic nuclei.
Here we overcome this problem using a machine-learning model, that is sufficiently flexible to describe the effect of the excess electron on the structure of the surrounding water, without including the electron in the model explicitly. The resulting potential is not onlyable to reproduce the stable cavity structure, but also recovers the correct lo-calization dynamics starting from neat water. The machine-learning model achieves the accuracy of the state-of-the-art correlated wave function method (second-order Møller-Plesset perturbation theory) it is trained on. It is sufficiently inexpensive to afford a full quantum statisticaland dynamical description, and allows us to achieve a highly accurate determination of the structure, diffusion mechanisms and vibrational spectroscopyof the solvated electron.
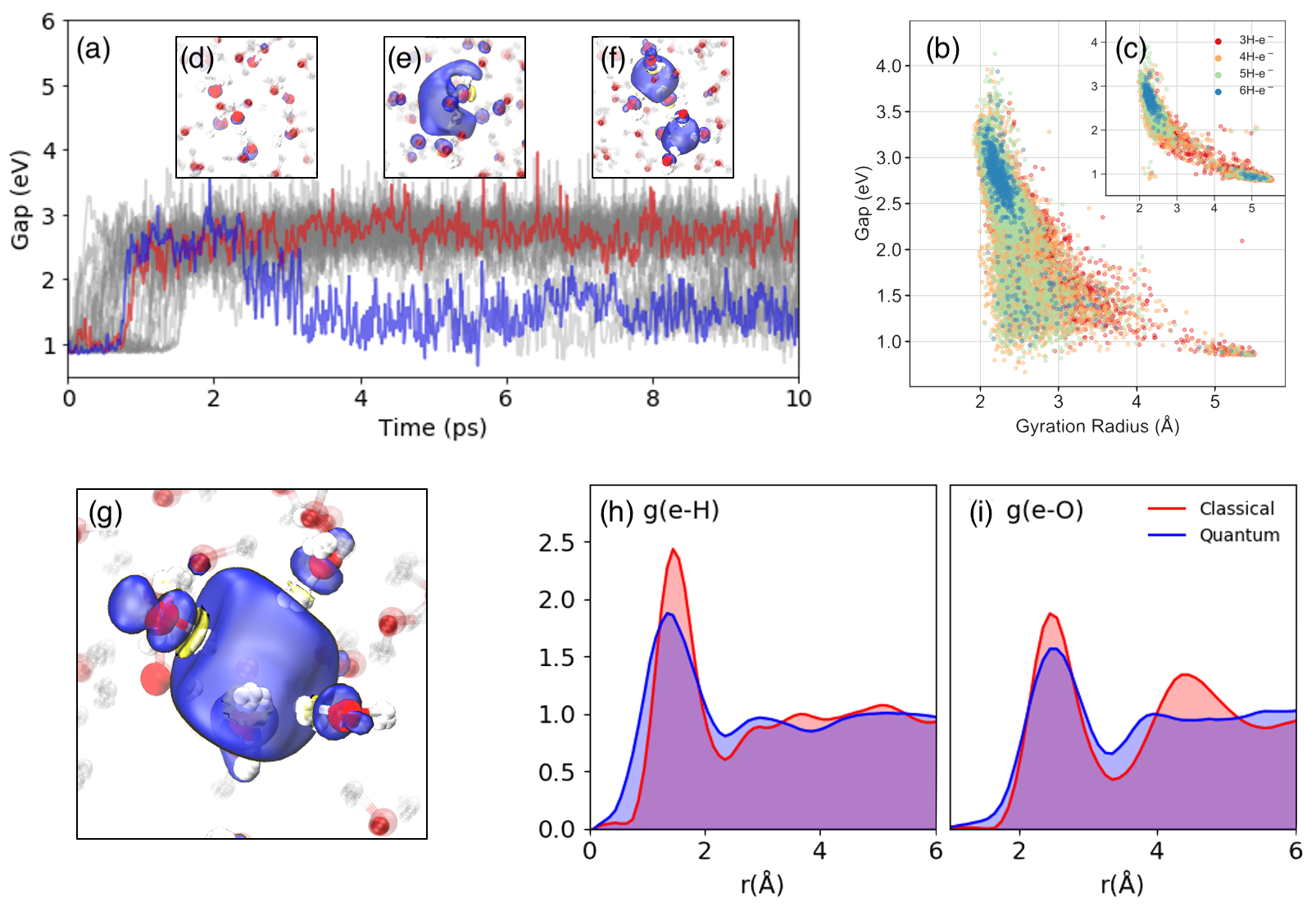
Figure: a) Time evolution of the gap of the excess electron. The representative evolution curves are marked in red (only involved with single-cavity) and blue (also involved twin-cavity). (b) Band gaps, gyration radii of the spin density distribution with different solvated electron coordination, as obtained from the quantum simulations. (c) distribution as obtained from classical simulations. The coordination number of solvated electron with respect to hydrogen are marked in red (3 H atoms), orange (4 H atoms), yellow (5 H atoms) and green (6 H atoms). Subplots (d,e,f): Spin densities of the solvated electron from quantum dynamics showing (d) the delocalized electron (e) the pre-solvated electron (f) a twin-cavity (g) a single cavity. (h,i) RDFs of hydrogen (h) and oxygen (e) atoms wrt to center of the solvated electron from classical (red) and quantum (blue) molecular dynamics.