High-affinity glycomimetic ligands for human Siglec-8
We present here the identification of glycomimetics with low µM affinity towards the human Siglec-8. Siglec-8 is an immunoglobulin-type lectin solely expressed on eosinophils and mast cells, and weakly on basophils. Many pathological conditions are associated with altered functions and/or numbers of these cells, among which allergic inflammation and asthma1. Despite the only partially known biological mechanism of action, the pharmacological importance of Siglec-8 has been demonstrated as eosinophil apoptosis and inhibition of mast cell degranulation could be achieved by means of anti-Siglec-8 monoclonal antibodies or synthetic glycopolymers decorated with Siglec-8 ligands2. However, no small molecules targeting Siglec-8 have been described so far. Such molecules could be useful to better elucidate the apoptotic cellular pathway and potentially provide a new pharmacological approach for eosinophil and mast cell associated diseases.
The glycan epitope recognized by Siglec-8 is the tetrasaccharide 6’-sulfo sialyl Lewisx (6’S-sLex)3. While the sialic acid carboxylate and the sulfate group on the galactose are involved in two crucial salt bridges, fucose and glucosamine show minor contributions to binding. Therefore, not surprisingly, we discovered that the related disaccharide Neu5Ac-Gal6S represents the minimal binding epitope (Fig. 1). This disaccharide served as lead compound for our search of new ligands with improved affinity and drug-like properties. In addition, it has been recently reported that sulfonamide modifications at the 9-position of the sialic acid moiety lead to compounds with increased activity4.
Applying different strategies, such as Gal6S replacement with non-carbohydrate moieties, bioisostere modifications, and extension of the glycerol side chain (Fig. 1), we synthesized a new series of glycomimetic structures. The best representative exhibits a low µM affinity, i.e. an almost 20-fold improved affinity compared to tetrasaccharide 6’S-sLex. ITC measurements revealed that binding of 6’S-sLex is punished with a substantial entropic penalty, whereas the disaccharide mimetics exhibit beneficial entropic and enthalpic contributions.
Our study made available potent small-molecule Siglec-8 antagonists, which can be used to further explored the biological role of Siglec-8.
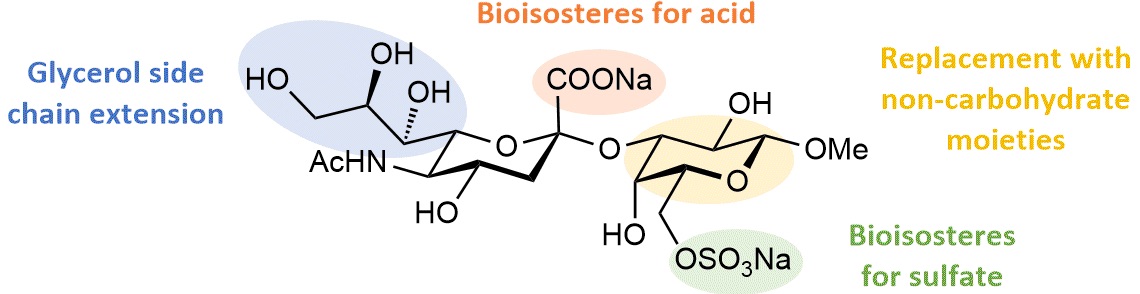
Figure 1. Chemical structure of Neu5Ac-Gal6S and the various modifications exploited for the discovery of high-affinity ligands.
[1] H. Floyd, J. Ni, A. L. Cornish, Z. Zeng, D. Liu, K. C. Carter, J. Steel, P. R. Crocker; J. Biol. Chem. 2000, 275, 861-866. [2] a) S. A. Hudson, N. V. Bovin, R. L. Schnaar, P. R. Crocker, B. S. Bochner; J. Pharmacol. Exp. Ther. 2009, 330, 608-612; b) B. A. Youngblood, E. C. Brock, J. Leung, R. Falahati, B. S. Bochner, H. S. Rasmussen, K. Peterson, C. Bebbington, N. Tomasevic; JCI Insight 2019, 4, e126219. [3] J. M. Pröpster, F. Yang, S. Rabbani, B. Ernst, F. H.-T. Allain, M. Schubert; PNAS 2016, 113, E4170-E4179. [4] C. M. Nycholat, S. Duan, E. Knuplez, C. Worth, M. Elich, A. Yao, J. O’Sullivan, R. McBride, Y. Wei, S. M. Fernandes, Z. Zhu, R. L. Schnaar, B. S. Bochner, J. C. Paulson; J. Am. Chem. Soc. 2019, 141, 14032-14037.