Synthetic Collagen Heterotrimers by Conformational Design
Collagen is a key structural protein with a unique triple helical fold that comprises three protein chains. Collagen heterotrimers are composed of two or three different strands and assume essential biological functions by binding to transmembrane receptors via recognition motifs that are defined by the spatial arrangement of the strands.[1] Synthetic access to heterotrimers allows for determining unknown chain arrangements of natural collagen and for developing collagen-like materials for biomedical applications.[1,2] The synthesis of heterotrimers is, however, challenging since three different strands (A, B, C) can self-assemble into 33 = 27 triple helices of different composition and chain arrangement (ACB, AAB, CBC, etc.).[2] To achieve assembly of distinct peptides into only one triple helix, amino acids that selectively interact with each other need to be incorporated into neighboring strands. So far, Lys and Asp residues have been utilized to direct heterotrimer formation by salt bridges between the ammonium and carboxylate groups, but due to the conformational flexibility of the Lys side-chain these interactions are rather weak.[2]
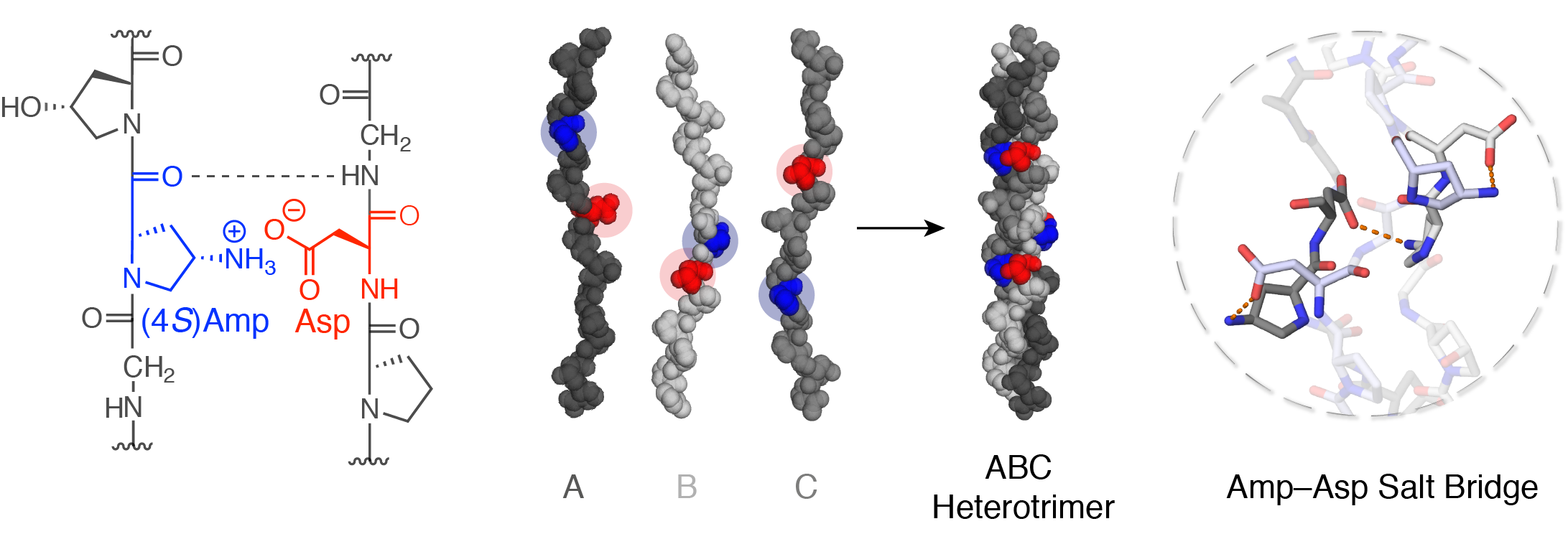
Here, we present a salt bridge between (4S)-aminoproline (Amp) and Asp that guides heterotrimer formation.[3] As a result of the rigid conformation of Amp, the ammonium group forms a geometrically defined salt bridge with the carboxylate group of Asp in an adjacent strand of the triple helix. Three Amp–Asp pairs proved sufficient to assemble a unique 24-mer ABC triple helix with complete selectivity over the 26 competing helices. The position of the Amp and Asp residues can readily be adjusted to access any other type of heterotrimer (AAB, ABA, etc.). Our design was corroborated by an X-ray crystallographic structure of an ABB heterotrimer that provided detailed structural insights into heterotrimeric triple helices and, importantly, contributed to the understanding of the optimal conformation of a salt bridge between ammonium and carboxylate groups.
In summary, our study enables the rational design of heterotrimeric collagen with full structural control. These results open intriguing prospects for the development of synthetic collagen for biological applications.
[1] Birgit Leitinger, Annu. Rev. Cell Dev. Biol. 2011, 27, 265–290.
[2] Abhishek A. Jalan, Jeffrey D. Hartgerink, Curr. Opin. Chem. 2013, 17, 960–967.
[3] Nina B. Hentzen, Valdrin Islami, Martin Köhler, Renato Zenobi, Helma Wennemers, J. Am. Chem. Soc. 2020, 142, 2208–2212.