A multi-modality platform to develop supramolecular radiopharmaceuticals
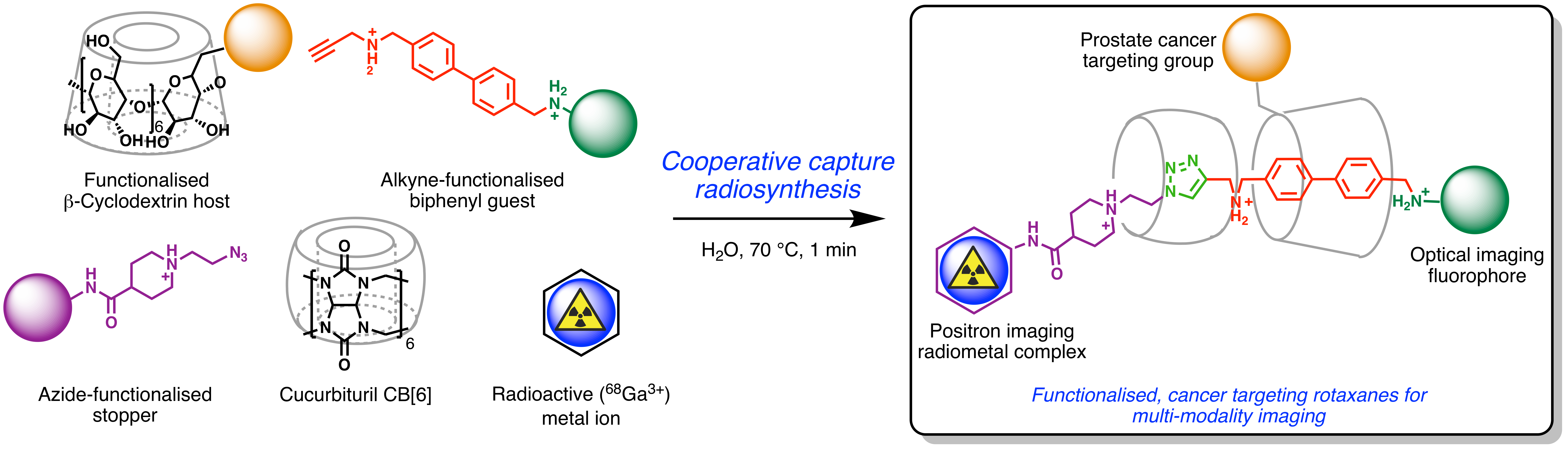
Supramolecular chemistry involves systems held together by non-covalent interactions. These systems hold promise in the design of drug delivery systems targeting cancer. Our goal is to use the supramolecular chemistry of rotaxanes as a scaffold for building multi-modality imaging agents. To this end, we report the synthesis and characterisation of functionalised, cancer targeting radiotracers.
A cooperative capture approach was used to develop radiolabelled, mechanically interlocked molecules (rotaxanes). Our attention focused on a ‘click’ chemistry reaction involving triazole formation by catalysis using CB[6] and β-CD. An efficient preorganisation between the azide and alkyne derivatives, CB[6] and β-CD via hydrogen bonds, facilitated a rapid synthesis of rotaxanes functionalised with metal ion complexes, fluorophores and cancer-specific ligands. The monofunctionalisation of β-CD also expand the versatility of the construct. To demonstrate the flexibility of our design, we synthesised various radioactive rotaxanes in a one-pot strategy. Thus, 68Ga-desferrioxamine or 68Ga-NODAGA complexes, fluorescein and Lys-NHC(O)NH-Glu inhibitors were used as stoppers on the axels of the rotaxanes or as functional features on the β-CD macrocycle. Reactions to make multi-functional rotaxanes were complete in less than one minute at 70 °C in biologically compatible media. All constructs were characterised by multinuclear NMR, high-resolution electrospray ionisation mass spectrometry and high-performance liquid chromatography. Radiolabelling reactions gave 68Ga-rotaxanes in high radiochemical yield and purity. To the best of our knowledge, this work represents the first use of supramolecular chemistry to access cancer-specific radiotracers for multi-modality imaging. Biological studies including cellular uptake and binding assays, and PET imaging in mouse models of human cancer, are underway.
[1] M. Webber, R. Langer, Chem. Soc. Rev., 2017, 46, 6600-6620.
[2] W. L. Mock, T. A. Irra, J. P. Wepsiec, T. L. Manimaran, J. Org. Chem., 1983, 48, 3619-3620.
[3] X. Hou, C. Ke, J. F. Stoddart, Chem. Soc. Rev., 2016, 45, 3766-3780.