Surface over-layer formation and dynamics in the strong metal-support interaction state on supported metal catalysts
In the early days of catalysis research, oxide carriers were considered to be mere inert carriers for highly dispersed metal nanoparticles. Forty years ago, experiments with noble metal-TiO2 catalysts demonstrated that oxide supports are far from inert in such catalytic systems and can strongly effect the adsorption behavior of catalysts and the phenomenon was named the strong metal-support interaction (SMSI). SMSI was observed in noble metal (NM) catalysts supported on reducible oxides such as TiO2. This phenomenon is of paramount importance, since the adsorption behavior of molecules on catalysts is a key factor in determining the catalytic activity. The SMSI phenomenon has, for the most part, been characterized ex situ or by means of model systems.
Here, complementary in situ TEM, ambient pressure X-ray Photoemission Spectroscopy (APXPS) and in situ synchrotron X-ray diffraction (XRD), supported by theoretical density functional theory (DFT) modelling, are used derive a holistic view of the SMSI formation mechanism. The results of this study identify the dual role of hydrogen in the formation of the SMSI state and elucidated the role of oxygen, which had not been recognized before. The migration of reduced titanium oxide, limited in thickness, and the formation of an alloy occur as competing mechanisms during reduction (Fig. 1 A–D). Subsequent exposure to oxygen segregates the titanium from the alloy, and a thicker TiOX overlayer forms (Fig. 1 E–H). If exposed again to hydrogen the alloy formation and partial surface layer reduction state is re-stabilized. This new understanding of the formation and dynamics of oxide layers on actual powder catalysts, is an important aid to resolve disagreement about the formation mechanism of the SMSI state.
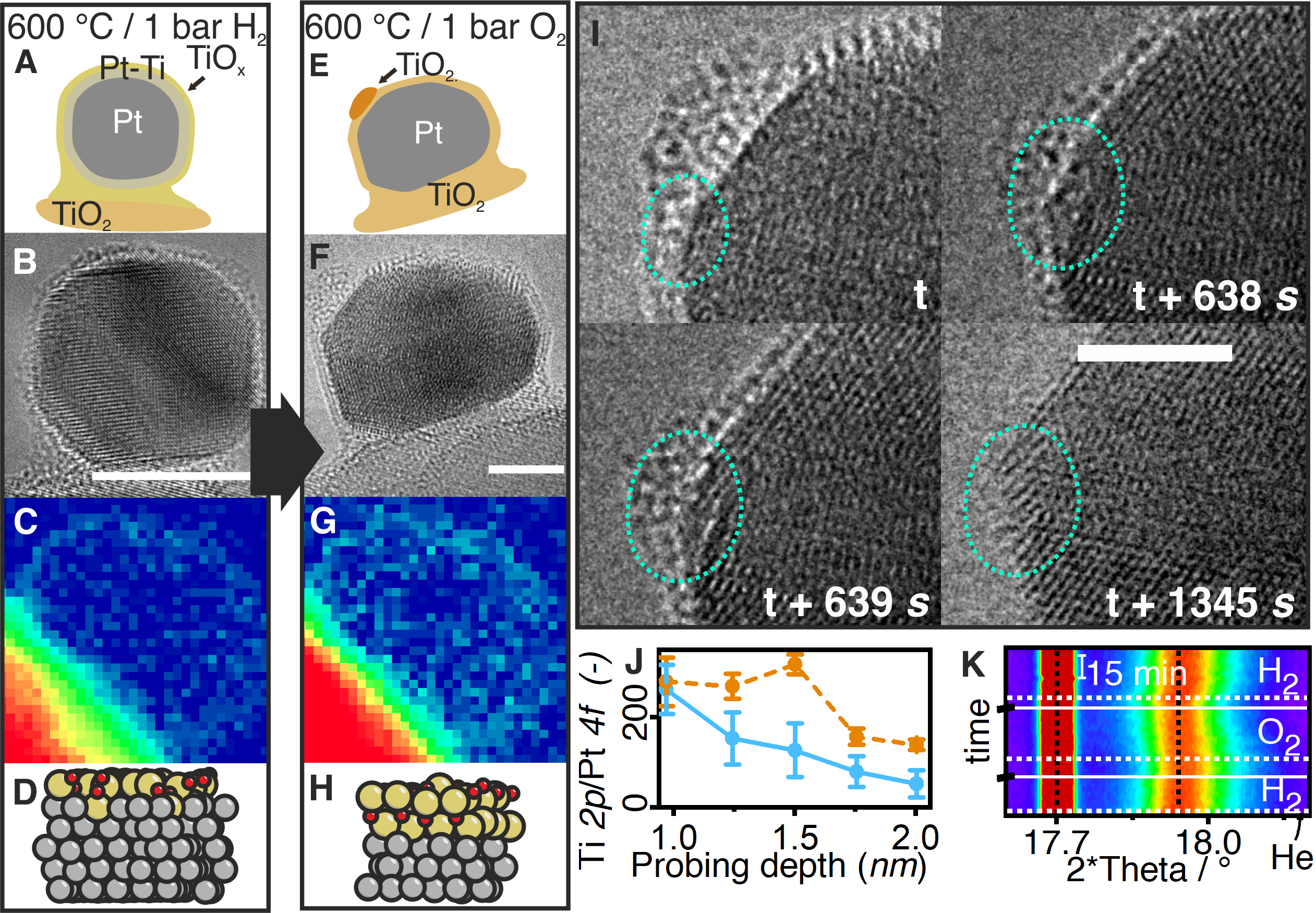
Figure 1: Schematic of the evolution of the SMSI state (A, E); in situ TEM (B, F); EELS mapping of the Ti L2,3-edge (C, G), most stable DFT surface model 1 monolayer (ML) TiO + 1 ML Pt8Ti on Pt (D) in H2 and 2 ML TiO2 on Pt (H) in O2; dynamics observed with in situ TEM when switching from O2 to H2 at 600 °C (I). Depth profiling derived from APXPS and in situ XRD (K) at 17.5 keV revealing the reflection shift of Pt at 17.9° upon gas switches.
[1] Tauster S.J., Fung S.C., Garten R.L. J. Am. Chem. Soc., 100, 1 (1978). / [2] Bernal S., Calvino J. et al. Catal. Today, 50, 2 (1999). / [3] Dulub O., Hebenstreit W., Diebold U. Phys. Rev. Lett., 84, 16 (2000).