Divergent Total Synthesis of (‒)-epicoccin G and (‒)-rostratin A Enabled by Double C(sp3)‒H Activation
Dithiodiketopiperazine (DTP) natural products comprise a large number of metabolites, which display a wide range of biological activities including antiviral, antibacterial, antiallergic, antimalarial and cytotoxic properties.[1] DTPs, characterized by sulfur atoms on a fused diketopiperazine (DKP) structure, have gained significant interest from the synthetic community, due to their unique structural and biological properties. In particular, the groups of Nicolaou, Reisman and Tokuyama have reported elegant total syntheses of DTP molecules containing a symmetrical pentacyclic ring system.[2,3,4] The innovative strategy reported herein is based on a Pd0-catalysed double C(sp3)‒H alkenylation key step allowing straightforward, high-yielding and concise access to a common advanced intermediate bearing the pentacyclic DKP scaffold.[5,6] The latter can be readily derivatised into several DTP natural products.
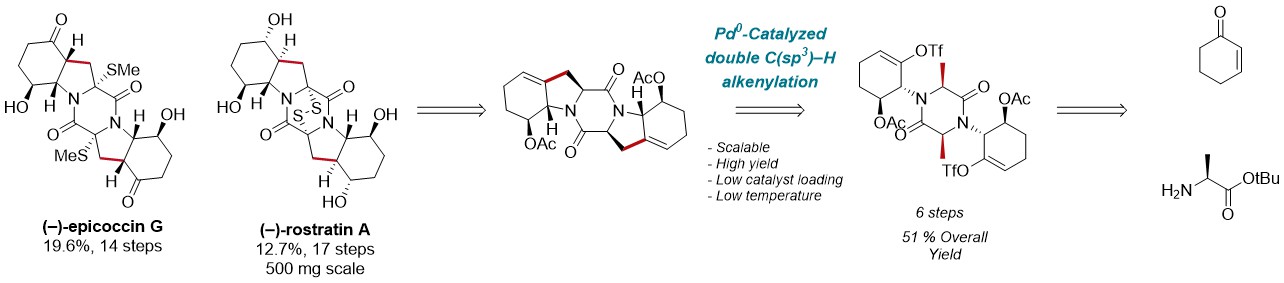
Herein, we report the application of this C(sp3)–H activation-based strategy to the enantioselective, scalable and divergent synthesis of (‒)-epicoccin G and (‒)-rostratin A, which are synthesized for the second and first time, respectively.[7,8] Moreover, in reason of their interesting cytotoxic properties, biological assays are currently undergoing on (‒)-rostratin A and closely related analogues.
[1] A. D. Borthwick, Chem. Rev. 2012, 112, 3641−3716.
[2] K. C. Nicolaou and al, J. Am. Chem. Soc. 2012, 134, 17320–17332.
[3] J. A. Codelli, A. L. A. Puchlopek and S. E. Reisman, J. Am. Chem. Soc. 2012, 134, 1930–1933
[4] H. Fujiwara, T. Kurogi, S. Okaya, K. Okano and H. Tokuyama, Angew. Chem., Int. Ed., 2012, 51, 13062–13065.
[5] O. Baudoin, Acc. Chem. Res., 2017, 50 (4), 1114–1123
[6] D. Dailler, G. Danoun, O. Baudoin, Angew. Chem. Int. Ed. 2015, 54, 4919-4922
[7] P. Thesmar, O. Baudoin, J. Am. Chem. Soc. 2019, 141, 15779−15783.
[8] P. Thesmar, S. Coomar, A. Prescimone, D. Gillingham, O. Baudoin, manuscript in preparation.