Alkali-Metal-Mediated Ferration (AMMFe): Synthetic and Structural Studies with Fluoroarenes and Beyond
Deprotonative metallation constitutes one of the most powerful methods to functionalise aromatic molecules. These reactions are usually performed by polar s-block metal bases such as lithium amides or alkyllithiums.[1] Previous studies have shown that building bimetallic bases combining an alkali-metal with a lower polarity main group metal, like Zn or Mg, it is possible to promote low polarity metallations.[2]
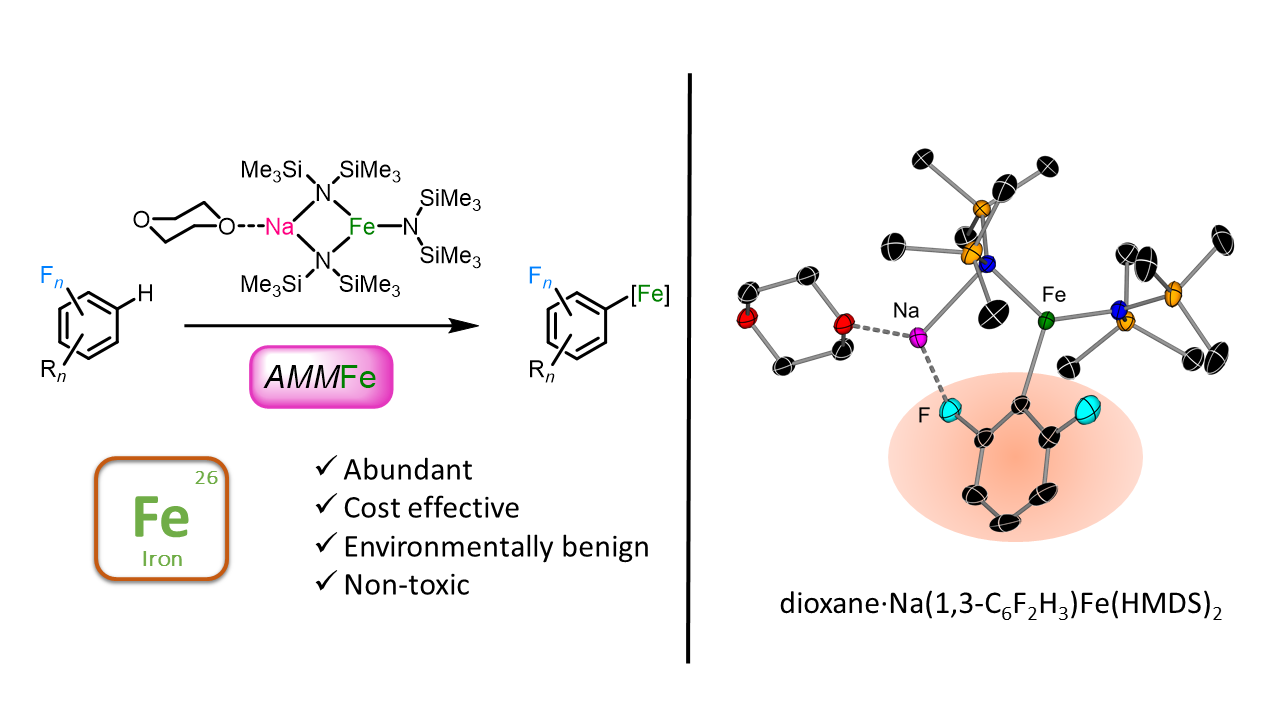
Expanding cooperative bimetallic chemistry into new territory, this contribution will report the first examples of direct ferration of sensitive fluoroaromatics.[3] By pairing sodium and iron (II) within the same molecular scaffold a powerful base has been developed, which enables direct Fe-H exchange reactions to occur selectively and in excellent yields. Key aspects controlling this unique reactivity, including the modus operandi of this Na/Fe bimetallic partnership, the key role of the alkali-metal as a facilitator of the ferration process as well as the influence of solvent effects will also be discussed.
[1] M. Schlosser in Organometallics in Synthesis: A Manual (Ed.: M. Schlosser), Wiley, Chichester, 2002, 1–352.
[2] R. E. Mulvey, F. Mongin, M. Uchiyama, Y. Kondo, Angew. Chem. Int. Ed. 2007, 46, 3802–24.
[3] L. C. H. Maddock, T. Nixon, A. R. Kennedy, M. R. Probert, W. Clegg, E. Hevia, Angew. Chem. Int. Ed. 2018, 57, 187–191.