Radiochemistry at PSI and the Chemistry of Transactinide Elements
About a third of all existing elements today do not have a stable isotope and are thus radioactive (see Fig. 1). Furthermore, if we take a look at all known isotopes of all known elements, a vast majority of more than 90% are in fact radioactive (see Fig. 1). Chemistry experiments with these radionuclides are subject of nuclear and radiochemistry. The corresponding, diverse activities in Switzerland range from fundamental topics regarding the chemistry of transactinide elements or the determination of high-precision nuclear data, such as half-lives or neutron capture cross sections, to more applied fields like nuclear waste separation and disposal, research on generation IV nuclear power reactors, radiopharmaceutical sciences and nuclear forensics. Here a short overview of the radiochemical activities of the Laboratory of Radiochemistry at the Paul Scherrer Institute (PSI) is presented together with a second part focusing on chemistry experiments with transactinide elements, i.e., the newest members of the periodic table of elements. The chemical characterization of these extraordinary elements allows today’s scientist to follow in the footsteps of earliest chemists such as D. I. Mendeleev and colleagues. The gathered results with these new elements contribute to a generally better understanding of how so-called relativistic effects govern physicochemical properties of heavy elements.
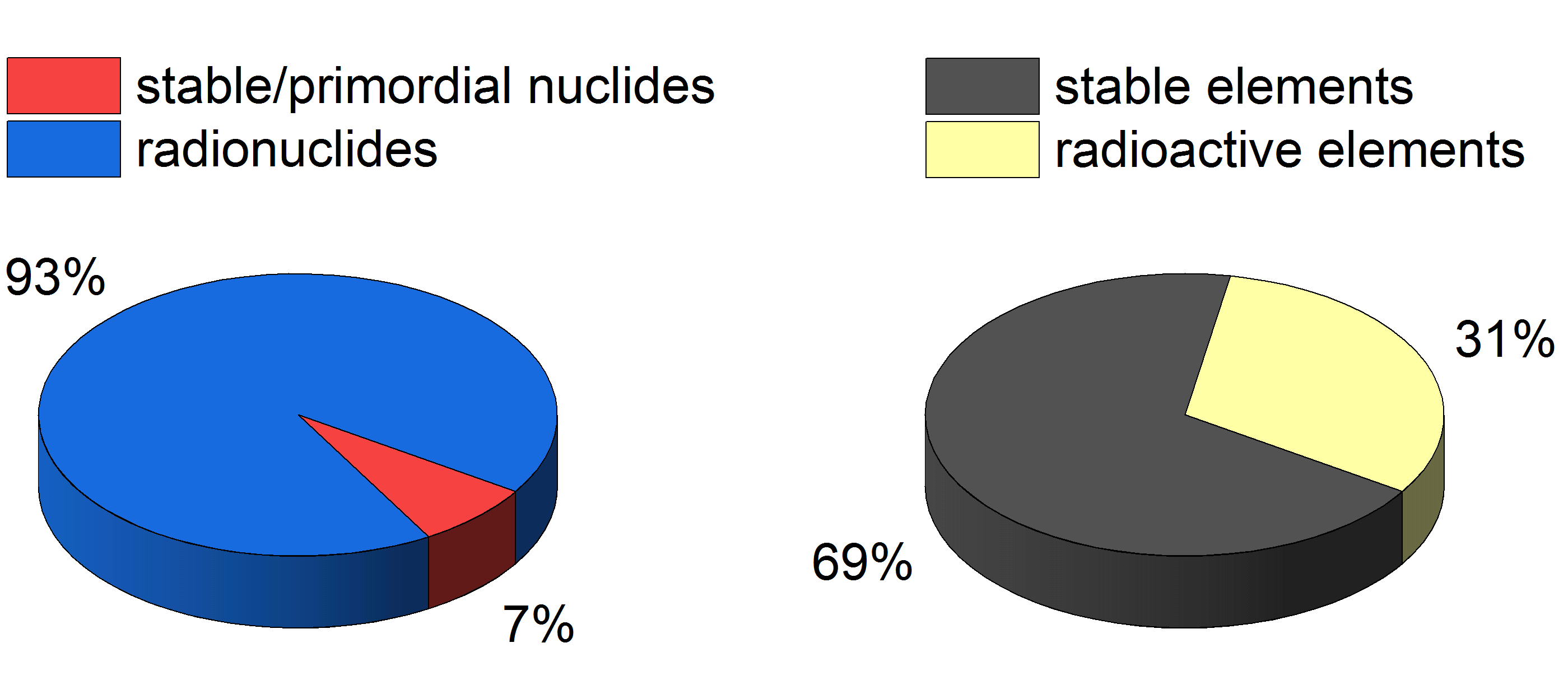
Figure 1: Pie charts of stable/primordial nuclides versus radionuclides (A) and stable elements versus radioelements (B).