Radical Functionalization of Cyclopropenes for the Synthesis of Bicyclo[3.1.0]hexanes and Substituted Alkenes
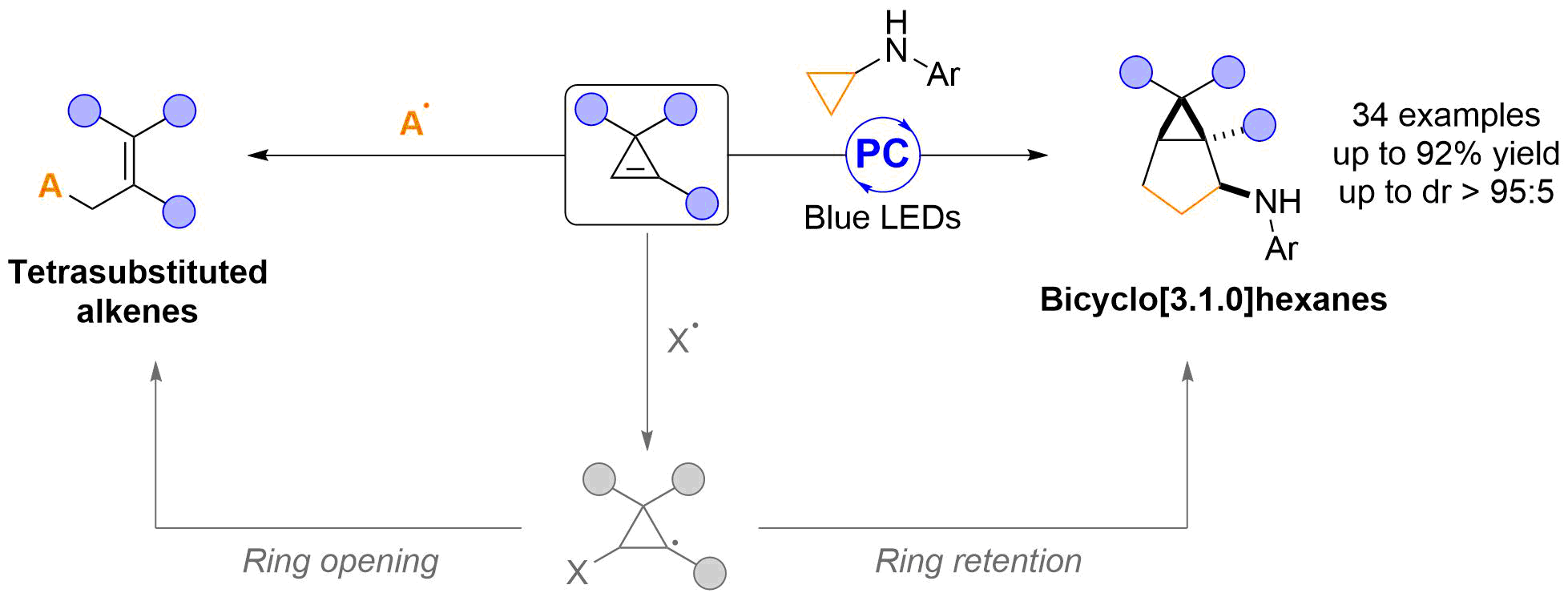
In the last two decades, cyclopropenes have been widely used as highly reactive three-carbon building blocks to access diverse chemical motifs. [1] Compared to carbometallation reactions and transition metal-mediated processes, [2] the use of radical chemistry to functionalize the double bond of cyclopropenes has not been much investigated so far, despite its high synthetic potential. [3] Herein, we report two new transformations based on the radical functionalization of cyclopropenes. The first proceeds with retention of the three-membered ring, involving a photoredox-mediated (3+2) annulation with cyclopropylanilines. This process provides a new and convergent strategy towards diastereomerically enriched and highly substituted bicyclo[3.1.0]hexanes, which are important scaffolds in medicinal chemistry. [4] In addition, our latest results for the synthesis of tetrasubstituted alkenes by a radical functionalization of cyclopropenes followed by ring opening, will be presented. [5] Discussion about this research will be also possible during a zoom meeting on Tuesday 25.08.2020, 12:15-13:15. [6]
[1] R. Vincente, Synthesis, 2016, 48, 2343-2360.
[2] Z. Zhu, Y. Weib, M. Shi, Chem. Soc. Rev., 2011, 40, 5534-5563.
[3] N. S. Dange, F. Robert, Y. Landais, Org. Lett., 2016, 18, 6156-6159.
[4] B. Muriel, A. Gagnebin, J. Waser, Chem. Sci., 2019, 10, 10716-10722.
[5] B. Muriel, J. Waser, Manuscript in preparation.
[6] The link will be available on the following document shortly before: https://drive.google.com/file/d/1s1Tg48ABj5q010FyUV29sckTHGopQv6o/view?usp=sharing